Recombinant COVID-19 Vaccine (Sf9 cell)
[NAME OF THE MEDICINAL PRODUCT] Recombinant COVID-19 Vaccine (Sf9 cell)
[ADJUVANT] Aluminum hydroxide
[THERAPEUTIC INDICATION] The product is indicated for active immunization to prevent coronavirus (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
[STRENGTHS] Each vial contains 1.0 mL. Single dose of 1.0 mL contains 40 μg of recombinant SARS-CoV-2 S-RBD protein.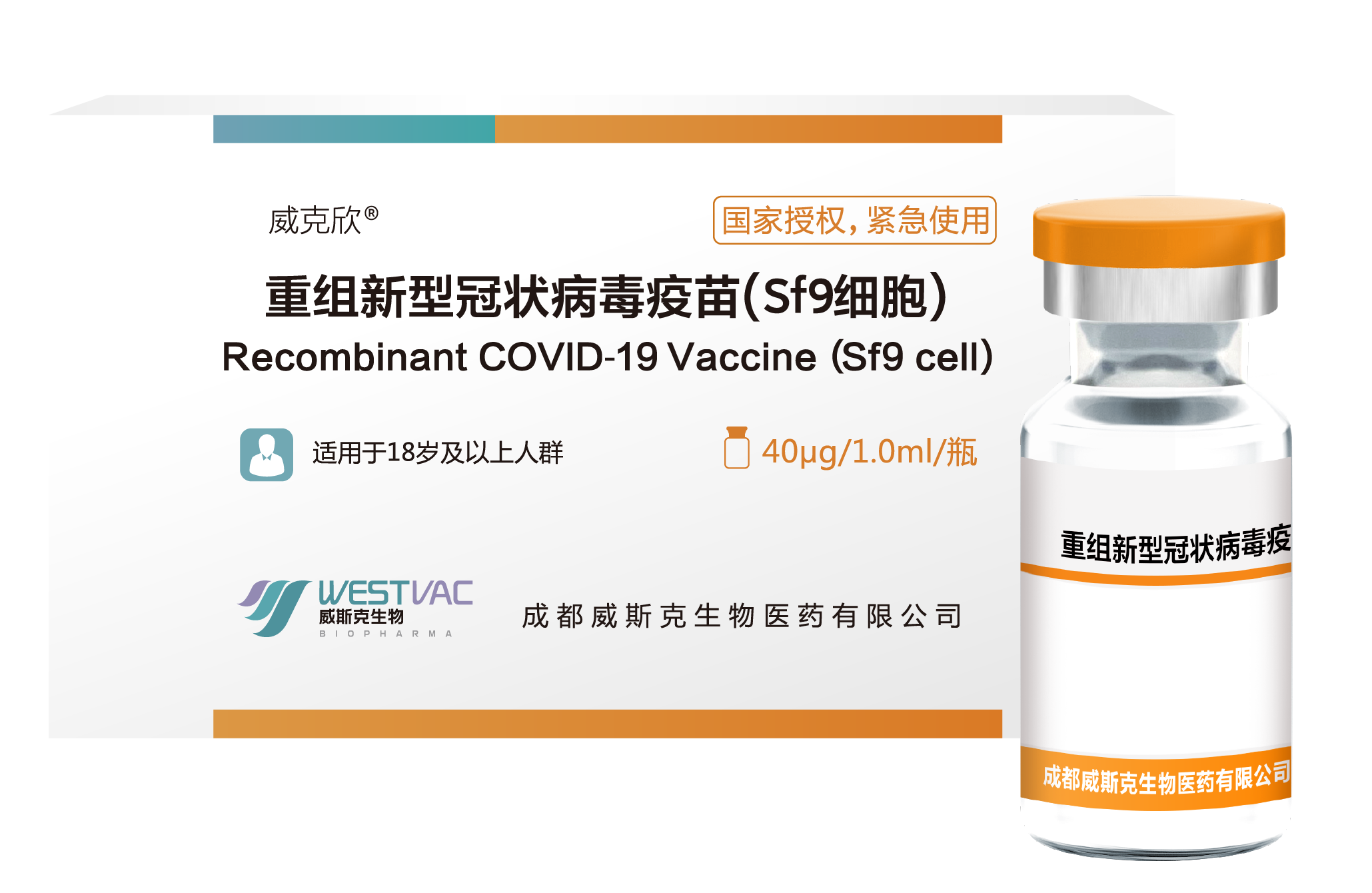
On December 2, 2022, it was approved by the relevant national authorities for emergency use.
Recombinant COVID-19 Vaccine (Sf9 cell) Package Insert
